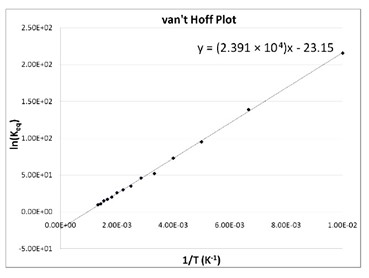
The van't Hoff Equilibrium Box is here depicted at the beginning of... | Download Scientific Diagram
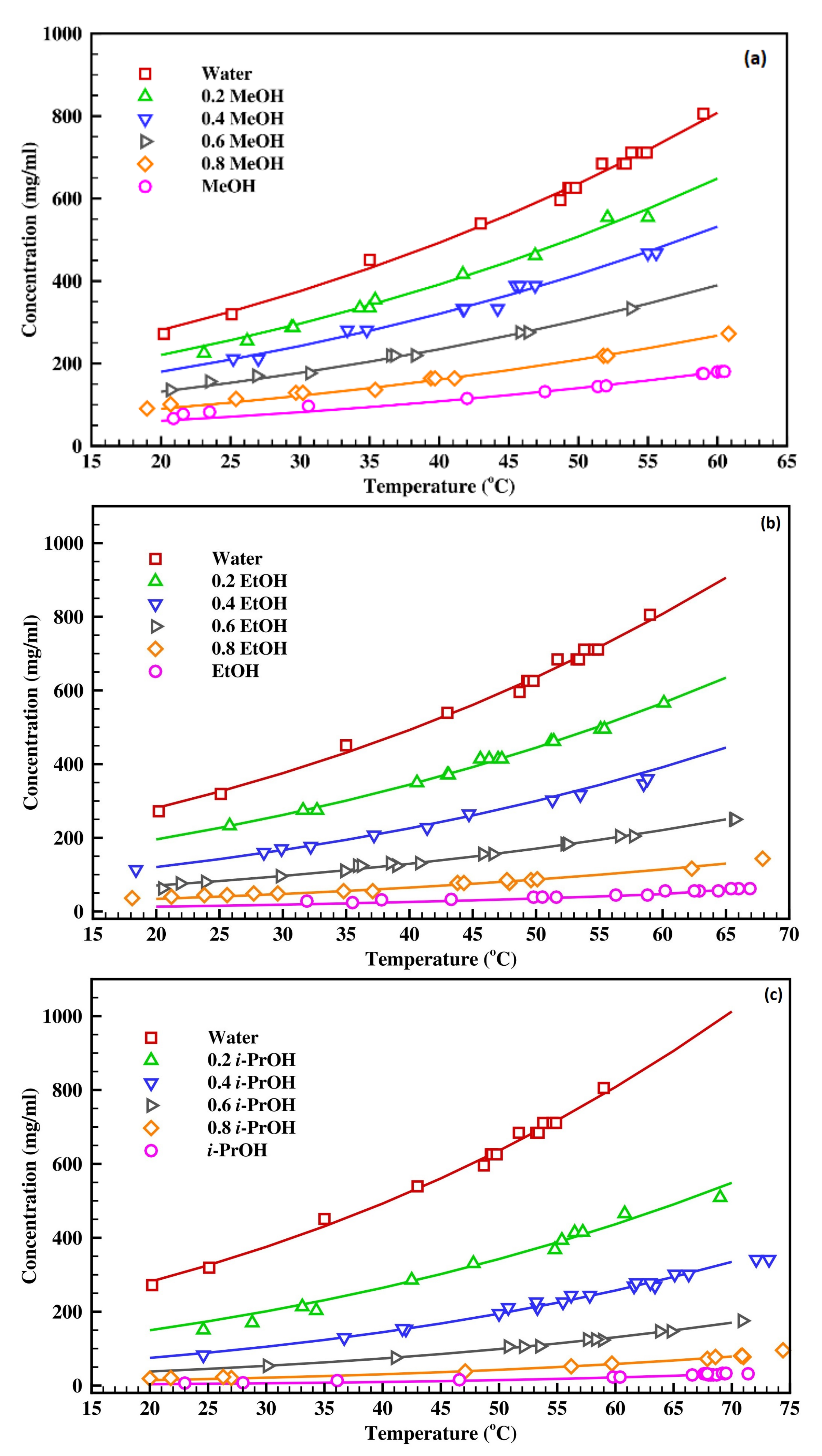
Crystals | Free Full-Text | Effect of Solvent Composition on Solubility, Thermodynamics, Metastable Zone Width (MSZW) and Crystal Habit of L-Ascorbic Acid

Crystals | Free Full-Text | Effect of Solvent Composition on Solubility, Thermodynamics, Metastable Zone Width (MSZW) and Crystal Habit of L-Ascorbic Acid
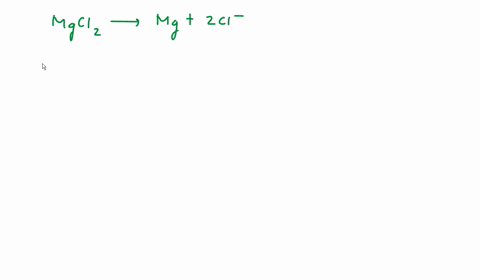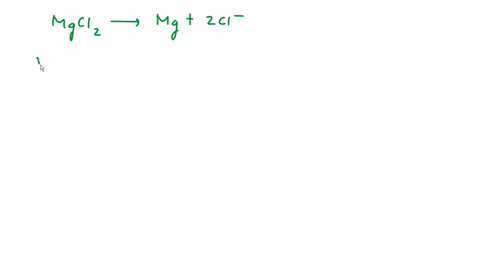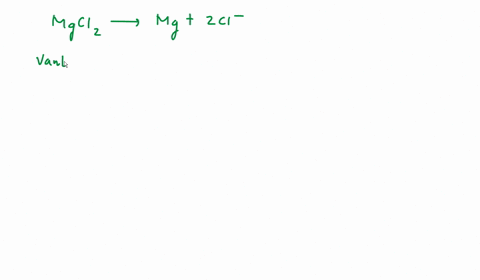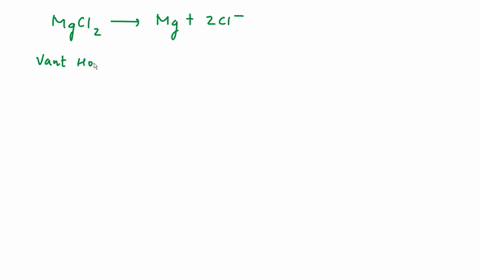
SOLVED: We often assume that the van't Hoff factor is simply equal to the number of ions present when the electrolyte dissociates in solution. In reality, van't Hoff factors are generally smaller
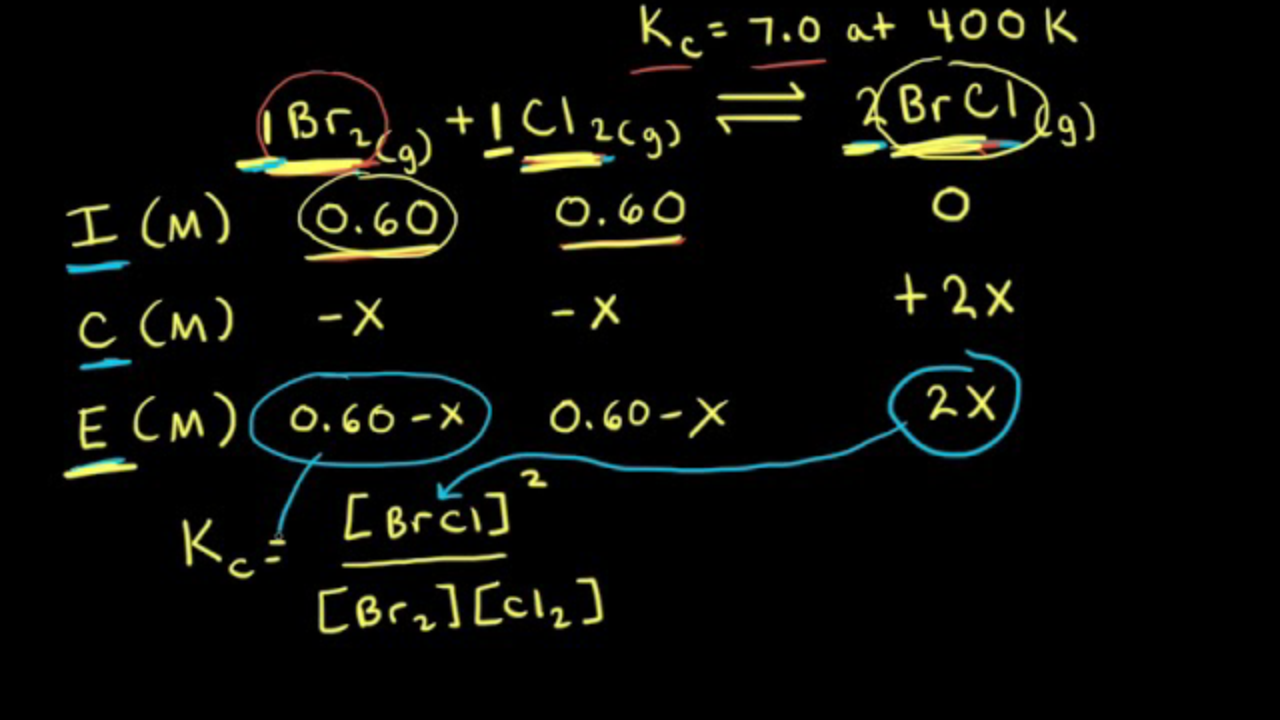
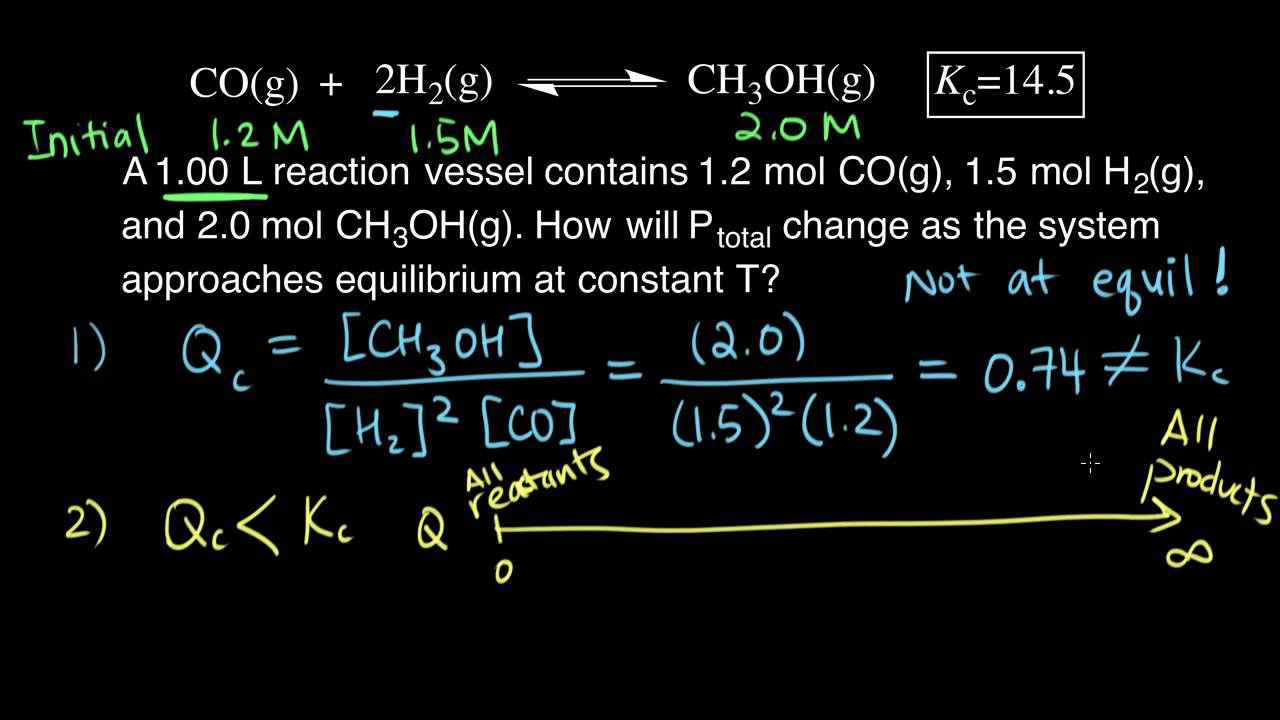
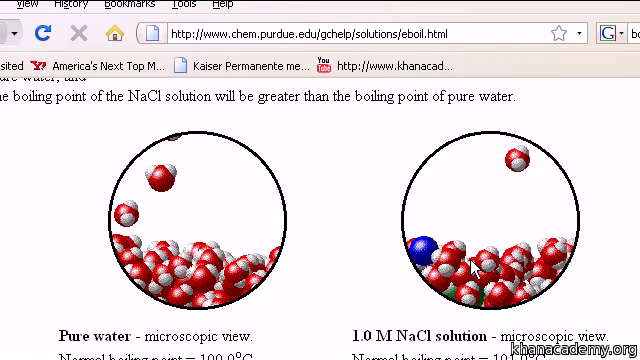
Calculating equilibrium concentrations from initial concentrations and the equilibrium constant (worked example) (video) | Khan Academy

Crystals | Free Full-Text | Effect of Solvent Composition on Solubility, Thermodynamics, Metastable Zone Width (MSZW) and Crystal Habit of L-Ascorbic Acid
What is the van 't Hoff factor for the NaCl in aqueous solution (assuming the complete dissociation of NaCl)? - Quora
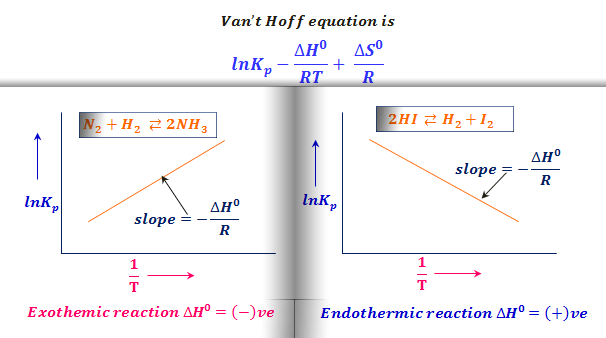
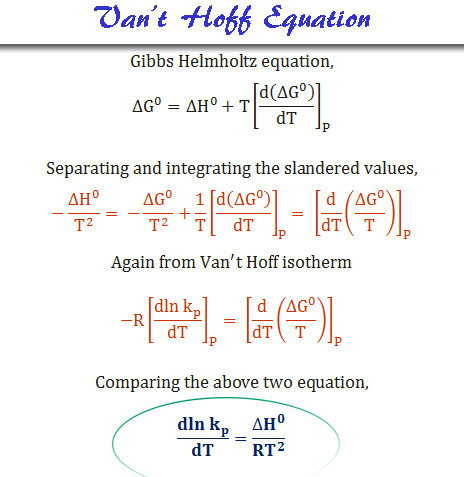
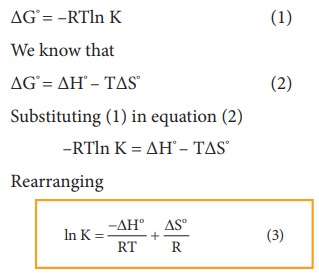
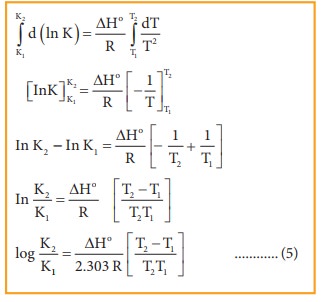
Van't Hoff equation-equilibrium. How much Van't Hoff equation — effect… | by Chemistry Topics | Medium